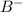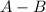Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 21:00
Harvey kept a balloon with a volume of 348 milliliters at 25.0˚c inside a freezer for a night. when he took it out, its new volume was 322 milliliters, but its pressure was the same. if the final temperature of the balloon is the same as the freezer’s, what is the temperature of the freezer? the temperature of the freezer is kelvins.
Answers: 2

Chemistry, 22.06.2019 07:50
Which of the following electromagnetic waves can create ions?
Answers: 2

Chemistry, 22.06.2019 11:50
If oil spills continue, all of the following should be expected except (2 points) death of aquatic life. polluted groundwater. decreased soil productivity. increased global temperatures.
Answers: 3

Chemistry, 22.06.2019 22:40
Covalent bonds generally form when the bonded elements have a difference in electronegativity less than 1.5. subtract the electronegativities for the following pairs of elements and predict whether they form a covalent bond. electronegativity difference of c and c: ionic covalent electronegativity difference of mg and cl: ionic covalent
Answers: 1
You know the right answer?
What is the definition of a lewis acid? a substance that accepts electrons to form a covalent bond...
Questions


Mathematics, 04.06.2021 03:50

Mathematics, 04.06.2021 03:50


Health, 04.06.2021 03:50


Mathematics, 04.06.2021 03:50

Mathematics, 04.06.2021 03:50



Mathematics, 04.06.2021 03:50




Biology, 04.06.2021 03:50

Chemistry, 04.06.2021 03:50


Biology, 04.06.2021 03:50


 +
+