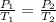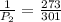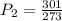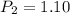Chemistry, 28.01.2020 13:27 conyers234
One mole of an ideal gas is sealed in a 22.4-l container at a pressure of 1 atm and a temperature of 273 k. the temperature is then increased to 301 k , but the container does not expand. what will the new pressure be?

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 15:50
The density of glycerin is 1.26grams/centimeter cubed . how many is this? use the conversion rates of and . express your answer to the correct number of significant figures.
Answers: 1

Chemistry, 22.06.2019 11:00
What is the molar mass of a gas that has density of 2.054 g/l
Answers: 2


Chemistry, 22.06.2019 18:00
Mercury turns to vapor at 629.88 k how much heat is lost 175 g of mercury vapor at 650 current condenses to a liquid at 297 ca mercury turns to weber at 629.88 kelvin how much he is lost 175 g of mercury vapor and 650 coming condensers to liquidate 297 kevin
Answers: 2
You know the right answer?
One mole of an ideal gas is sealed in a 22.4-l container at a pressure of 1 atm and a temperature of...
Questions


Biology, 22.01.2020 02:31

Mathematics, 22.01.2020 02:31


Biology, 22.01.2020 02:31


Mathematics, 22.01.2020 02:31

History, 22.01.2020 02:31

Mathematics, 22.01.2020 02:31



Biology, 22.01.2020 02:31




Spanish, 22.01.2020 02:31


History, 22.01.2020 02:31