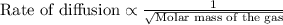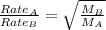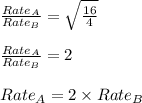Chemistry, 13.11.2019 13:31 zaniyastubbs9
Asample of gas a has a molar mass of 4 grams while a sample of gas b has a molar mass of 16 grams. which statement holds true?
both gas a and gas b diffuse at the same speed.
gas a effuses faster than gas b.
gas b effuses faster than gas a.
the molar masses of gas a and gas b are not related to effusion.
the molar mass is directly proportional to the rate of effusion.

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 22:30
Imagine that you’re getting ready to move to a new city. when people move, they are influenced by push factors and pull factors, and you have many reasons for your move. which of the following factors is an example of a pull factor? a. wanting to move because you’ve found a great new school somewhere new b. needing to move because there are not enough resources in your old hometown c. being forced to move because your old home is gone d. having to move because there are no jobs in your current hometown
Answers: 1


Chemistry, 22.06.2019 03:20
What is the ima of the 1 st class lever in the graphic given? 2 3 0.5
Answers: 1

Chemistry, 22.06.2019 18:00
An object displaces 652 ml of water. the volume of the object is: 0.652 cm³ 6.52 cm³ 65.2 cm³ 652 cm³
Answers: 2
You know the right answer?
Asample of gas a has a molar mass of 4 grams while a sample of gas b has a molar mass of 16 grams. w...
Questions


English, 11.10.2019 19:10

Physics, 11.10.2019 19:10

Physics, 11.10.2019 19:10


Mathematics, 11.10.2019 19:10

Mathematics, 11.10.2019 19:10

English, 11.10.2019 19:10




Social Studies, 11.10.2019 19:10


Biology, 11.10.2019 19:10