
Chemistry, 14.11.2019 14:31 meganpaughstu
Oxygen has a density of 1.43 g/l at stp. what is the density of oxygen at 1.30 atm?

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 22:30
Explain why scientists use shared characteristics to make cladograms.
Answers: 1

Chemistry, 21.06.2019 23:50
How will the emission of an alpha particle affect the atomic number of an atom
Answers: 3

Chemistry, 22.06.2019 03:30
Select the correct answer. when carbon dioxide dissolves in water, it sometimes reacts with water to form carbonic acid as in this balanced equation: co2 + h2o → h2co3. if 495 milliliters of carbon dioxide at 25°c and 101.3 kilopascals reacts with excess water, what is the theoretical yield of carbonic acid? use the periodic table and the ideal gas resource a. 0.889 g b. 1.10g c. 1.27g d. 2.02g what's the answer! quick!
Answers: 1

Chemistry, 22.06.2019 15:00
Many ionic compounds and a few highly polar covalent compounds are because they completely ionize in water to create a solution filled with charged ions that can conduct an electric current.
Answers: 1
You know the right answer?
Oxygen has a density of 1.43 g/l at stp. what is the density of oxygen at 1.30 atm?...
Questions

Biology, 01.09.2021 20:40


Mathematics, 01.09.2021 20:40


Social Studies, 01.09.2021 20:40


Mathematics, 01.09.2021 20:40


Arts, 01.09.2021 20:40




Mathematics, 01.09.2021 20:40



Business, 01.09.2021 20:40

Mathematics, 01.09.2021 20:40

Mathematics, 01.09.2021 20:40

Mathematics, 01.09.2021 20:40

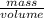
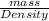 ....... (2)
....... (2)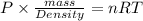
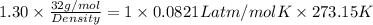
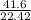 g/L
g/L


