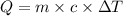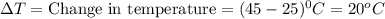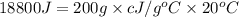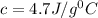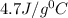Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 15:50
Elements in group 2 are all called alkaline earth metals. what is most similar about the alkaline earth metals?
Answers: 1

Chemistry, 22.06.2019 17:00
The arrangement of particles is most ordered in a sample of
Answers: 1


Chemistry, 23.06.2019 07:30
Using this reversible reaction, answer the questions below: n2o4 2no2 (colorless) (reddish-brown) -as the temperature increased, what happened to the n2o4 concentration? -was the formation of reactants or products favored by the addition of heat? -which reaction is exothermic? right to left or left to right? -if the change of enthalpy of this reaction when proceeding left to right is 14 kcal, which chemical equation is correct? n2o4 2no2 + 14 kcal n2o4 2no2, hr = +14 kcal n2o4 + 14 kcal 2no2 n2o4 2no2, hr = -14 kcal
Answers: 1
You know the right answer?
Two hundred grams of a substance requires 18.8 kj of heat to raise its temperature from 25°c to 45°c...
Questions


Mathematics, 10.11.2019 16:31

Mathematics, 10.11.2019 16:31

Business, 10.11.2019 16:31




Mathematics, 10.11.2019 16:31


Spanish, 10.11.2019 16:31

Biology, 10.11.2019 16:31



Physics, 10.11.2019 16:31





History, 10.11.2019 17:31

Mathematics, 10.11.2019 17:31