
Chemistry, 29.01.2020 08:00 kellynadine02
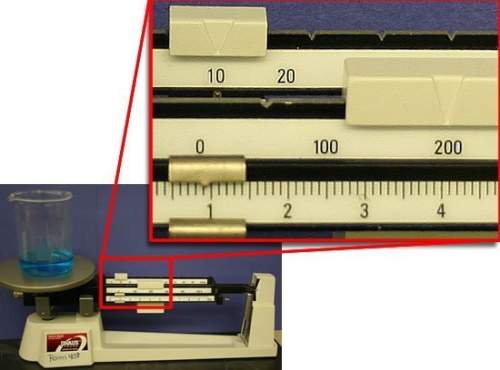
what is the mass of water in the beaker? assume that the beaker has a mass of exactly 100.00 g.
a) 100.90 g
b) 109.00 g
c) 110.90 g
d) 210.90 g

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 20:30
An exothermic reaction is conducted in an insulated calorimeter filled with water. the calorimeter is then sealed so that there is no heat exchanged between the contents of the container and the surrounding air. which of the following statements is true about the reaction?
Answers: 1

Chemistry, 22.06.2019 08:00
Joe shines white light into a bowl half full of water at an angle of incident of 27.5°. calculate the angle of refraction in the water given the indices of refraction for air and water are 1.00 and 1.36, respectively.
Answers: 2

Chemistry, 22.06.2019 22:20
How do cfcs cause ozone depletion? how do cfcs cause ozone depletion? ultraviolet radiation breaks down cfcs, molecules containing chlorine. chlorine then breaks one oxygen atom away from ozone, leaving behind a paired oxygen molecule. ultraviolet radiation breaks down cfcs, molecules containing chlorine. chlorine then breaks two oxygen atoms away from ozone, leaving behind a paired oxygen molecule. ultraviolet radiation creates cfcs, molecules containing chlorine. chlorine then breaks two oxygen atoms away from ozone, leaving behind a paired oxygen molecule. ultraviolet radiation creates cfcs, molecules containing chlorine. chlorine then breaks one oxygen atom away from ozone, leaving behind a paired oxygen molecule.
Answers: 2

Chemistry, 22.06.2019 22:30
What relationship exists between an enzyme and a catalyst?
Answers: 1
You know the right answer?
what is the mass of water in the beaker? assume that the beaker has a mass of exactly 100.00 g.
Questions

Mathematics, 23.04.2020 03:18




English, 23.04.2020 03:18

English, 23.04.2020 03:18

Mathematics, 23.04.2020 03:18




Mathematics, 23.04.2020 03:18


Mathematics, 23.04.2020 03:18


Mathematics, 23.04.2020 03:18

English, 23.04.2020 03:18






