
Chemistry, 07.12.2019 19:31 kealinwiley
If all the n2 and h2 are consumed, what volume of nh3, at the same temperature and pressure, will be produced? at a certain temperature and pressure, 1.9 l of n2 reacts with 5.7 l of h2.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 01:30
Arollercoaster car at the top of a hill has potential energy kinetic energy chemical energy light energy
Answers: 1

Chemistry, 22.06.2019 09:20
What happened to the amount of carbon dioxide in the atmosphere from 2010–2017?
Answers: 1

Chemistry, 22.06.2019 20:30
We are hoping to create 5.72 grams of glucose. the plant was given 4.75 liters of co2 and 2.81 g of h20. which reactant was the limiting reagent? how much excess mass did we have of the other reactant?
Answers: 2

Chemistry, 22.06.2019 23:00
What is a substance? a. a physical property of matter b. a chemical property of matter c. an element or compound that cannot be physically separated d. characteristics used to tell the difference between mixtures
Answers: 1
You know the right answer?
If all the n2 and h2 are consumed, what volume of nh3, at the same temperature and pressure, will be...
Questions










English, 26.09.2019 03:10

History, 26.09.2019 03:10





History, 26.09.2019 03:10

Mathematics, 26.09.2019 03:10

History, 26.09.2019 03:10


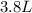 of
of 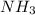
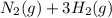 ⇒
⇒ 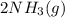
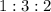 (I)
(I)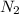 and with 3 moles of
and with 3 moles of 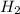 we will obtain 2 moles of
we will obtain 2 moles of 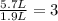 (II)
(II)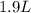 of
of 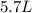 of
of 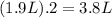 of
of 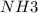 (two times more liters that
(two times more liters that 

