
Chemistry, 05.10.2019 20:00 angellinelittle
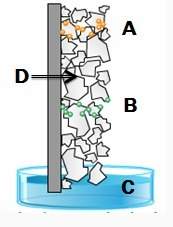
All chromatography experiments require both a stationary phase and mobile phase. in this thin -layer chromatography experiment, which is the stationary phase?
a) a
b) b
c) c
d) d

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 07:40
The formation of a solid, also known as a is an indication of a chemical change. precipitate particulate particle powder
Answers: 3

Chemistry, 22.06.2019 14:00
The content of manganese (mn) in steel was determined spectrophotometrically and with the use of the standard addition method. an unknown sample of mn from a digested steel sample gave an absorbance of 0.185 when analyzed spectrophotometrically. when 5.00 ml of solution containing 95.5 ppm mn was added to 50.0 ml of the unknown steel solution (digested sample), the absorbance was 0.248. calculate the concentration, in parts-per-million (ppm), of mn in the digested steel sample solution.
Answers: 3

Chemistry, 22.06.2019 18:10
Consider the following reaction at equilibrium: c(s)+h2o(g)⇌co(g)+h2(g) predict whether the reaction will shift left, shift right, or remain unchanged upon each of the following disturbances. a) c is added to the reaction mixture. b) h2ois condensed and removed from the reaction mixture c) co is added to the reaction mixture d) h2 is removed from the reaction mixture.
Answers: 3

Chemistry, 22.06.2019 19:00
Mercury metal is poured into a graduated cylinder that holds exactly 22.5 ml the mercury used to fill the cylinder mass in 306.0 g from this information calculate the density of mercury
Answers: 2
You know the right answer?
All chromatography experiments require both a stationary phase and mobile phase. in this thin -layer...
Questions

Mathematics, 28.05.2020 02:03

Biology, 28.05.2020 02:03

Mathematics, 28.05.2020 02:03


Mathematics, 28.05.2020 02:03

Mathematics, 28.05.2020 02:03

Mathematics, 28.05.2020 02:03

Mathematics, 28.05.2020 02:03

English, 28.05.2020 02:03


Health, 28.05.2020 02:03


Mathematics, 28.05.2020 02:03



Mathematics, 28.05.2020 02:03



Medicine, 28.05.2020 02:03

History, 28.05.2020 02:03



