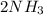Chemistry, 30.09.2019 00:30 markaylarowland8
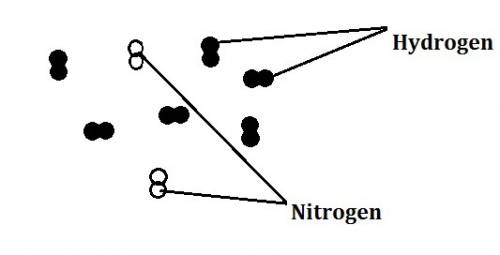
In the haber process, nitrogen (n2) and hydrogen (h2) are directly combined to form ammonia (nh3). which illustration contains the stoichiometric quantities of the reactants for this reaction?

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 07:30
According to the vsepr theory what is the shape of a molecule that has a central atom valence three other items with no lone pairs of electrons
Answers: 1

Chemistry, 22.06.2019 18:10
Given the following at 25c calculate delta hf for hcn (g) at 25c. 2nh3 (g) +3o2 (g) + 2ch4 (g) > 2hcn (g) + 6h2o (g) delta h rxn= -870.8 kj. delta hf=-80.3 kj/mol for nh3 (g), -74.6 kj/mol for ch4, and -241.8 kj/mol for h2o (g)
Answers: 1

Chemistry, 22.06.2019 21:00
The rate constant for the reaction below is 6.2 x 10−5 mol l−1 s −1. if the initial concentration of a is 0.0500 m, what is its concentration after 115 s?
Answers: 1

Chemistry, 23.06.2019 00:00
Total the mass on the syringe. record it in the correct row of the data table. kg done click and drag weights to change the pressure. click the syringe to zoom in and see the volume. intro
Answers: 3
You know the right answer?
In the haber process, nitrogen (n2) and hydrogen (h2) are directly combined to form ammonia (nh3). w...
Questions

Mathematics, 21.09.2021 20:40


English, 21.09.2021 20:40



Mathematics, 21.09.2021 20:50

Mathematics, 21.09.2021 20:50


English, 21.09.2021 20:50




Health, 21.09.2021 20:50

Mathematics, 21.09.2021 20:50



Chemistry, 21.09.2021 20:50



Business, 21.09.2021 20:50

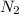 +
+ 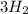 =
=