
Chemistry, 09.11.2019 21:31 natfloresm13
Salicylic acid (c7h6o3) reacts with acetic anhydride (c4h6o3) to form acetylsalicylic acid (c9h8o4).
2c7h6o3(aq) + c4h6o3(aq) mc002-1.jpg 2c9h8o4(aq) + h2o(l)
what is the limiting reactant if 70.0 g of c7h6o3 and 80.0 g of c4h6o3 react?
a water
b salicylic acid
c acetic anhydride
d acetylsalicylic acid

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 20:30
Un cierto gas tiene un volumen de 800ml a 80°c y 600ml a 80°c y 600mmhg de presión. ¿cual será el volumen del gas a condiciones normales? sí el gas es oxígeno, ¿cuál será su peso? y ¿cuántas moléculas están presentes en el sistema?
Answers: 2

Chemistry, 22.06.2019 15:20
Draw any one of the skeletal structures of a 2° alkyl bromide having the molecular formula of c6h13br and two stereogenic centers. indicate chirality by using wedge and hashed wedge notation. lone pairs do not need to be shown.
Answers: 1

Chemistry, 22.06.2019 18:30
What volume of a 0.0606 m solution of strontium bromide is needed to obtain 0.340 mol of the compound? question 42 options: a)5.61 l b) 3.4 l c) 600 ml d) 1 l e) 178 ml
Answers: 1

You know the right answer?
Salicylic acid (c7h6o3) reacts with acetic anhydride (c4h6o3) to form acetylsalicylic acid (c9h8o4)....
Questions


SAT, 09.12.2021 02:50

Mathematics, 09.12.2021 02:50

SAT, 09.12.2021 02:50


History, 09.12.2021 02:50

Chemistry, 09.12.2021 02:50

English, 09.12.2021 02:50



Computers and Technology, 09.12.2021 02:50



History, 09.12.2021 02:50



Biology, 09.12.2021 02:50



Mathematics, 09.12.2021 02:50



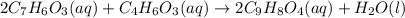
 mole of acetic anhydride
mole of acetic anhydride

