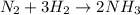Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 11:00
Imagine that twenty i.u.’s of enzyme z were catalyzing the above reaction for one minute, under vmaxconditions, in a 3.00 ml assay volume. the assay is buffered with 20 mm phosphate buffer, ph 7.60. what will the ph be at the end of that one minute?
Answers: 2

Chemistry, 22.06.2019 20:30
A40 kilogram skier starts at the top of a 12 meter high slope. at the bottom, she is travelling 10 meters per second. how much energy does she lose to friction
Answers: 2

Chemistry, 22.06.2019 21:50
28. which is not a reason that water is used to store spent fuel rods from nuclear power plants? water increases the speed of the chain reaction in the fuel rods. water protects nuclear power plant workers from the high temperature and radiation of the fuel rods. water acts as a radiation shield to reduce the radiation levels. water cools the spent rods. salts action
Answers: 1

Chemistry, 23.06.2019 01:00
Atoms contain subatomic particles called protons and neutrons. when these protons and neutrons spilt, a lot of energy is released
Answers: 3
You know the right answer?
An industrial process involves reacting nitrogen gas (n2 and hydrogen gas (h2 to produce ammonia (nh...
Questions

Mathematics, 12.12.2020 16:00

History, 12.12.2020 16:00

Mathematics, 12.12.2020 16:00



Mathematics, 12.12.2020 16:00

Spanish, 12.12.2020 16:00

Biology, 12.12.2020 16:00

Mathematics, 12.12.2020 16:00



Mathematics, 12.12.2020 16:00


Law, 12.12.2020 16:00

Business, 12.12.2020 16:00





Mathematics, 12.12.2020 16:00