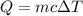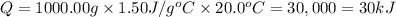Chemistry, 02.09.2019 02:20 izzyisawesome5232
Asample of octane (c8h18 that has a mass of 0.750 g is burned in a bomb calorimeter. as a result, the temperature of the calorimeter increases from 21.0°c to 41.0°c. the specific heat of the calorimeter is 1.50 j/(g °c, and its mass is 1.00 kg. how much heat is released during the combustion of this sample? use .

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 21:30
Which is the layer underground where all empty spaces are filled with a combination of air and water ?
Answers: 1

Chemistry, 23.06.2019 01:00
Which is true concerning the products and reactants of photosynthesis and cellular respiration? a. the products of photosynthesis are sugars and the reactants of cellular respiration are starches. b. the products of photosynthesis are reactants in cellular respiration. c. oxygen is needed for photosynthesis and is given off in cellular respiration.
Answers: 2

Chemistry, 23.06.2019 01:00
Wind and moving water provide energy. chemical mechanical thermal none of the above
Answers: 1

Chemistry, 23.06.2019 05:00
Match each term to its description. match term definition excess reactant a) reactant that can produce a lesser amount of the product limiting reactant b) amount of product predicted to be produced by the given reactants theoretical yield c) reactant that can produce more of the product
Answers: 3
You know the right answer?
Asample of octane (c8h18 that has a mass of 0.750 g is burned in a bomb calorimeter. as a result, th...
Questions


Mathematics, 08.02.2021 06:40

English, 08.02.2021 06:40


Mathematics, 08.02.2021 06:40

Mathematics, 08.02.2021 06:40





Mathematics, 08.02.2021 06:40

Biology, 08.02.2021 06:40

Spanish, 08.02.2021 06:40

Chemistry, 08.02.2021 06:40

Mathematics, 08.02.2021 06:40



Law, 08.02.2021 06:40


Chemistry, 08.02.2021 06:40