
Chemistry, 29.01.2020 22:57 hiitslillyhere
What type of reaction is shown in this thermochemical equation? a + b + heat → c + d
endothermic
combination
decomposition
exothermic

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 23:30
Start an single atom tab. observe the decay of polonium-211. after each decay, press the reset nucleus button to watch the process again. write a description of alpha decay for po-211
Answers: 2

Chemistry, 22.06.2019 10:30
What woukd most likely be the transmittance at a 0.70 m solution of solute a? a) 7.6%b) 1.1%c)4.0%d)4.6%
Answers: 1

Chemistry, 22.06.2019 20:00
Iam hoping to create 5.72 grams of glucose. the plant was given 4.75 liters of co2 and 2.81 g of h20. which reactant was the limiting reagent? how much excess mass did we have of the other reactant?
Answers: 1

You know the right answer?
What type of reaction is shown in this thermochemical equation? a + b + heat → c + d
endothe...
endothe...
Questions


Engineering, 08.08.2019 17:20


Social Studies, 08.08.2019 17:20


Computers and Technology, 08.08.2019 17:20

Engineering, 08.08.2019 17:20






Social Studies, 08.08.2019 17:20




Social Studies, 08.08.2019 17:20

Social Studies, 08.08.2019 17:20

Social Studies, 08.08.2019 17:20

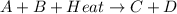 is an endothermic reaction.
is an endothermic reaction.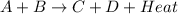 is an exothermic reaction.
is an exothermic reaction.

