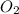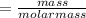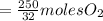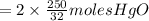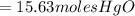Mercury(ii) oxide (hgo) decomposes to form mercury (hg) and oxygen (o2). the balanced chemical equation is shown below.
2hgo 2hg + o2
the molar mass of hgo is 216.59 g/mol. the molar mass of o2 is 32.00 g/mol. how many moles of hgo are needed to produce 250.0 g of o2?
3.906
7.813
15.63
73.87

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 07:30
What three things determine the shape and size of a puddle when water is poured out onto a surface
Answers: 2

Chemistry, 22.06.2019 20:00
What is the molar mass of the anhydrous compound? answer using four significant figures. 36.02 g/mol 120.15 g/mol 156.12 g/mol
Answers: 1

Chemistry, 23.06.2019 02:00
What causes the appearance of lines in a emission spectrum
Answers: 1

Chemistry, 23.06.2019 02:00
Scientists are often interested in knowing the molar heat of combustion – the heat released during the combustion of one mole of a substance. use the periodic table to find molar masses. how many moles of ethanol are present in the sample?
Answers: 2
You know the right answer?
Mercury(ii) oxide (hgo) decomposes to form mercury (hg) and oxygen (o2). the balanced chemical equat...
Questions




French, 12.01.2021 23:30


English, 12.01.2021 23:30


Biology, 12.01.2021 23:30



Computers and Technology, 12.01.2021 23:30

English, 12.01.2021 23:30


English, 12.01.2021 23:30

Mathematics, 12.01.2021 23:30


History, 12.01.2021 23:30


Mathematics, 12.01.2021 23:30