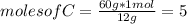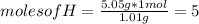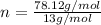Chemistry, 25.11.2019 03:31 GreenHerbz206
Asample of a compound contains 60.0 g c and 5.05 g h. its molar mass is 78.12 g/mol. what is the compound’s molecular formula?

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 09:40
Which diagram shows the correct way to represent an ionic compound of magnesium oxide?
Answers: 3

Chemistry, 22.06.2019 22:10
What is the indicator of the number of ions in solution? the amount of conductivity the amount of precipitate the amount of solute added
Answers: 1

Chemistry, 23.06.2019 00:30
When did stem cell research become known ? who discovered stem cell? what experiments or studies have been conducted so far?
Answers: 3

Chemistry, 23.06.2019 11:40
Which of the following observations indicates that an atom has neutrons? some uncharged particles are scattered by a beryllium atom when it hits a gold foil. some uncharged particles bounce back from a gold foil when it is bombarded with alpha particles. a radiation consisting of uncharged particles is emitted when alpha particles strike beryllium atoms. a radiation which attracts electrons is produced when a beryllium atom is bombarded with alpha particles.
Answers: 2
You know the right answer?
Asample of a compound contains 60.0 g c and 5.05 g h. its molar mass is 78.12 g/mol. what is the com...
Questions

History, 10.10.2020 19:01


Mathematics, 10.10.2020 19:01

Chemistry, 10.10.2020 19:01


Mathematics, 10.10.2020 19:01

Mathematics, 10.10.2020 19:01





Arts, 10.10.2020 19:01

Social Studies, 10.10.2020 19:01

Mathematics, 10.10.2020 19:01