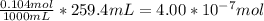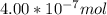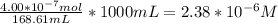Chemistry, 24.09.2019 12:00 divadebbgirl1
When a chemist titrates a standard solution of 168.61 ml of hydrochloric acid (hcl) with 0.104 m sodium hydroxide (naoh) , she finds that it requires 259.4 ml of the base to reach the endpoint of the titration. what is the molarity of the acid solution ?

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 21:30
How air particles exert a pressure on the inside of the balloon
Answers: 1


Chemistry, 22.06.2019 15:30
Two metal blocks that have slightly different temperatures are placed next to one another. after five minutes, they both have lower but equal temperatures. according to the law of conservation of energy, what most likelyhappened? energy was created inside the blocks.energy was destroyed inside the blocks.energy was absorbed into the blocks from outside the system.energy was transferred from the warmer block to the cooler block.
Answers: 2

Chemistry, 22.06.2019 16:00
Which of the following is the correct definition of chemical energy? a. energy an object has because of its motion or position b. energy resulting from the flow of charged particles, such as electrons or ions c. energy produced from the splitting of atoms d. energy stored in chemical bonds of molecules
Answers: 1
You know the right answer?
When a chemist titrates a standard solution of 168.61 ml of hydrochloric acid (hcl) with 0.104 m sod...
Questions

Mathematics, 25.08.2019 23:10




History, 25.08.2019 23:10







History, 25.08.2019 23:10



English, 25.08.2019 23:10


Spanish, 25.08.2019 23:10



Computers and Technology, 25.08.2019 23:10