
Chemistry, 24.11.2019 22:31 fortwill05
Rust results from iron’s reaction to oxygen. an iron nail gains mass when it rusts. how does this reaction support the law of conservation of mass?
a. the mass of the rusted nail equals the mass of iron and the oxygen from the air it reacted with to form the rust.
b. the mass of the rusted nail increases because iron attracts more protons from the air.
c. the increased mass of the rusted nail is an exception to the law of conservation of mass since the rusted nail’s mass increases.
d. the increased mass of the rusted nail results from the rearrangement of protons and neutrons within oxygen, according to the law of conservation of mass.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 09:30
Which element is the least metallic between cadmium, silver, zinc, or iron?
Answers: 1

Chemistry, 22.06.2019 10:00
Select all of the methods through which a drug can enter your body. injection swallowing inhalation absorption
Answers: 2

Chemistry, 22.06.2019 22:30
Which is a characteristic of the electron sea model for metallic bonding? molecular orbitals overlap to produce bands. electrons flow easily between metal nuclei. electrons are in fixed positions in the orbitals. atomic nuclei are arranged in an irregular pattern.
Answers: 3

Chemistry, 23.06.2019 03:30
Which of the following describes the entropy change as a solution is made from a liquid and solid
Answers: 1
You know the right answer?
Rust results from iron’s reaction to oxygen. an iron nail gains mass when it rusts. how does this re...
Questions

Mathematics, 13.12.2019 11:31


Biology, 13.12.2019 11:31





Mathematics, 13.12.2019 11:31

Mathematics, 13.12.2019 11:31



Mathematics, 13.12.2019 11:31


Mathematics, 13.12.2019 11:31

Mathematics, 13.12.2019 11:31

Chemistry, 13.12.2019 11:31


Biology, 13.12.2019 11:31


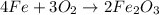
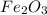 .
.

