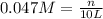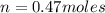Pcl5 => pcl3+cl2
the no of moles of cl2 produced will be if one mole of pcl5 is heated 25...


Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 03:10
Agas diffuses 1/7 times faster than hydrogen gas (h2). what is the molar mass of the gas? 100.10 g/mol 98.78 g/mol 86.68 g/mol 79.98 g/mol
Answers: 3

Chemistry, 22.06.2019 03:40
Chemical kinetics what was the rate of reaction in trial 3? choose the closest answer.
Answers: 3


Chemistry, 22.06.2019 10:00
According to the tide table below what time of day will the highest tide occur? (2 pt) the highest tide will occur at
Answers: 1
You know the right answer?
Questions



Physics, 27.09.2019 04:30

Social Studies, 27.09.2019 04:30


Mathematics, 27.09.2019 04:30


Mathematics, 27.09.2019 04:30

Mathematics, 27.09.2019 04:30




Business, 27.09.2019 04:30

Mathematics, 27.09.2019 04:30


Business, 27.09.2019 04:30

Mathematics, 27.09.2019 04:30


Mathematics, 27.09.2019 04:30

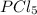 = 1 mole
= 1 mole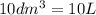
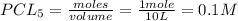
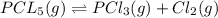
![K_c=\frac{[PCl_3][Cl_2]}{[PCl_5]}](/tpl/images/0394/8934/73fe0.png)
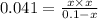
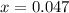
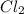 produced is 0.047 M
produced is 0.047 M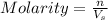
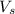 = volume of solution in L
= volume of solution in L