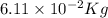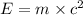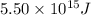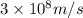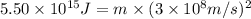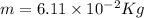The mass defect of a particular reaction resulted in the production of 5.50 x 10^15 joules of energy. what mass (if totally converted to energy) would correspond to this amount of energy? (1 j = 1 kg m^2/s^2)
1.83 x 10^8 kg
4.95 x 10^7 kg
6.11 x 10^-2 kg
1.64 x 10^-1 kg

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 15:00
‘which reaction would most likely require the use of an inert electrode?
Answers: 1

Chemistry, 22.06.2019 17:00
Which statement is true about a catalyst? a: a catalyst decreases the rate of the reaction. b. a catalyst is consumed during a chemical reaction. c. a catalyst lowers the activation energy of a reaction. d. a catalyst increases the reactant concentration during a reaction.
Answers: 1

Chemistry, 22.06.2019 17:00
The atoms of a solid aluminum can are close together, vibrating in a rigid structure. if the can is warmed up on a hot plate, what happens to the atoms?
Answers: 3

Chemistry, 23.06.2019 00:30
What are the advantages of using the metric system? designed as a decimal system making conversions simpler more accurate system of measurement has prefixes that correspond to an amount to use with all base units used by the entire scientific community
Answers: 2
You know the right answer?
The mass defect of a particular reaction resulted in the production of 5.50 x 10^15 joules of energy...
Questions


Mathematics, 17.12.2020 14:00



Mathematics, 17.12.2020 14:00

Mathematics, 17.12.2020 14:00

Mathematics, 17.12.2020 14:00




Social Studies, 17.12.2020 14:00


Mathematics, 17.12.2020 14:00


Social Studies, 17.12.2020 14:00




Mathematics, 17.12.2020 14:00

Mathematics, 17.12.2020 14:00