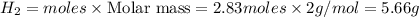Work the entire problem from the beginning. how many grams of h2 would be formed if 34 grams of carbon reacted with an unlimited amount of h2o? the reaction is: c + h2o → co + h2 the atomic mass of c is 12.01 g/mole. the atomic mass of h2 is 2.016 g/mole. grams of hydrogen

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 04:50
Compare the equilibrium constants for the systems shown in the table. which favors products the most? which favors products the least? rank these systems in order from most to least in terms of favoring products rather than reactants. d > b > a > c c > a > b > d b > c > d > a a > d > c > b
Answers: 1

Chemistry, 22.06.2019 06:40
Which statement is usually true about the relationship between activation energy and reaction rates? low activation energy barriers result in low rates. high activation energy barriers result in low rates. low activation energy barriers result in no reaction. high activation energy barriers result in no reaction.
Answers: 3

Chemistry, 22.06.2019 18:30
When a device is used in a circuit in which the voltage is 81 v the current flowing through the device is 3 a what is the resistance of the device
Answers: 2

Chemistry, 22.06.2019 19:30
Describe the forces both attractive and repulsive that occur as two atoms move closer together.
Answers: 1
You know the right answer?
Work the entire problem from the beginning. how many grams of h2 would be formed if 34 grams of carb...
Questions

Mathematics, 29.10.2020 05:00

Mathematics, 29.10.2020 05:00

Mathematics, 29.10.2020 05:00

Mathematics, 29.10.2020 05:00

History, 29.10.2020 05:00


Computers and Technology, 29.10.2020 05:00

Mathematics, 29.10.2020 05:00




History, 29.10.2020 05:00




Geography, 29.10.2020 05:00



French, 29.10.2020 05:00

Mathematics, 29.10.2020 05:00

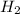 would be formed if 34 grams of carbon reacted with an unlimited amount of
would be formed if 34 grams of carbon reacted with an unlimited amount of 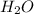
 of particles.
of particles.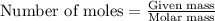
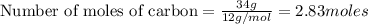
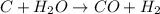
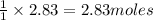 of
of