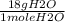Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 21:20
Phosgene (carbonyl chloride), cocl2, is an extremely toxic gas that is used in manufacturing certain dyes and plastics. phosgene can be produced by reacting carbon monoxide and chlorine gas at high temperatures: co(g) cl2(g)⇌cocl2(g) carbon monoxide and chlorine gas are allowed to react in a sealed vessel at 477 ∘c . at equilibrium, the concentrations were measured and the following results obtained: gas partial pressure (atm) co 0.830 cl2 1.30 cocl2 0.220 what is the equilibrium constant, kp, of this reaction
Answers: 2

Chemistry, 23.06.2019 06:30
The polarity of an oxygen-hydrogen bond is higher than the polarity of a nitrogen-hydrogen bond, allowing amines to be more soluble than alcohols.
Answers: 3

Chemistry, 23.06.2019 13:00
Write the balanced chemical reaction for the formation of fe2(so4)3 from fe2o3 and so3 and determine how many moles of fe2(so4)3 are formed when 12.7 mol of so3 are reacted.
Answers: 1

You know the right answer?
Calculate the mass of water produced when 7.14g of butane reacts with excess oxygen...
Questions

Physics, 25.09.2019 07:10




Mathematics, 25.09.2019 07:10

Mathematics, 25.09.2019 07:10



Biology, 25.09.2019 07:10

Mathematics, 25.09.2019 07:10

Physics, 25.09.2019 07:10



Social Studies, 25.09.2019 07:10

Mathematics, 25.09.2019 07:10





Advanced Placement (AP), 25.09.2019 07:10

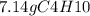 x
x 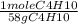 ×
×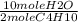 ×
×