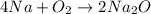a. the density of ice is 0.4 g/ml

Chemistry, 28.08.2019 18:00 willveloz4
Which statement describes a chemical property of matter?
a. the density of ice is 0.4 g/ml
b. the boiling point of ethanol is 78.4c
c. rust is a flaky material with an orange-red color
d. sodium is a very reactive element that combines easily with nonmetals

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 01:00
Look at the bean data from days 4–6. use these data to explain how natural selection changed the number of dark red walking beans over time. writing part
Answers: 3

Chemistry, 22.06.2019 04:30
Use the drop-down menus to answer each question. which runner finished the 100 m race in the least amount of time? which runner stopped running for a few seconds during the race? at what distance did anastasia overtake chloe in the race?
Answers: 1

Chemistry, 22.06.2019 07:20
After watching the video "zinc strip in copper nitrate solution", and reading the instructions, click on the link labeled "start" just below the drawing of the pencil tip. follow the direction to complete the 3x3 grid. answer the below questions for the portion of the activity in which sn(s) is placed in agno3(aq)
Answers: 1

Chemistry, 22.06.2019 12:00
Consider the following reaction at equilibrium. 2co2 (g) 2co (g) + o2 (g) h° = -514 kj le châtelier's principle predicts that the equilibrium partial pressure of co (g) can be maximized by carrying out the reaction a. at high temperature and high pressure b. at high temperature and low pressure c. at low temperature and low pressure d. at low temperature and high pressure e. in the presence of solid carbon
Answers: 2
You know the right answer?
Which statement describes a chemical property of matter?
a. the density of ice is 0.4 g/ml
a. the density of ice is 0.4 g/ml
Questions



Mathematics, 07.06.2020 13:57

English, 07.06.2020 13:57

English, 07.06.2020 13:57

Mathematics, 07.06.2020 13:57

Chemistry, 07.06.2020 13:57

World Languages, 07.06.2020 13:57

Mathematics, 07.06.2020 13:57

Biology, 07.06.2020 13:57

Mathematics, 07.06.2020 13:57


Social Studies, 07.06.2020 13:57

Computers and Technology, 07.06.2020 13:57

Mathematics, 07.06.2020 13:57



Mathematics, 07.06.2020 13:57

Chemistry, 07.06.2020 13:57

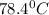 is a physical property.
is a physical property.