
Chemistry, 17.09.2019 09:30 karlaperez482
Use the equation 2c4h10 +13o2--> 8co2+10h2o to find how many moles of oxygen would react with .425 mol c4h10

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 02:00
In the following redox reaction which is the oxidizing agent and which is the reducing agent? alcl3 + na nacl + al
Answers: 1

Chemistry, 22.06.2019 02:30
98 ! and brainliest plz ! the below reaction can be categorized as more than one type of reaction. which reactions are these, and what are the types of reactions?
Answers: 1

Chemistry, 22.06.2019 07:30
Calculate the ratio of h+ ions to oh– ions at a ph = 7. find the concentration of h+ ions to oh– ions listed in table b of your student guide. then divide the h+ concentration by the oh– concentration. record this calculated ratio in table a of your student guide. compare your approximated and calculated ratios of h+ ions to oh– ions at a ph = 7. are they the same? why or why not? record your comparison in table a. what is the concentration of h+ ions at a ph = 7? mol/l what is the concentration of oh– ions at a ph = 7? mol/l what is the ratio of h+ ions to oh– ions at a ph = 7? : 1
Answers: 1

Chemistry, 22.06.2019 19:50
Which sentence from holes contains an implied personality trait? stanley and his parents had tried to pretend that he was just going away to camp for a while, just like rich kids do. he'd just been in the wrong place at the wrong time. stanley felt somewhat dazed as the guard unlocked his handcuffs and led him off the bus. stanley nodded to show he understood
Answers: 3
You know the right answer?
Use the equation 2c4h10 +13o2--> 8co2+10h2o to find how many moles of oxygen would react with .42...
Questions

Spanish, 15.01.2021 08:00

Computers and Technology, 15.01.2021 08:00




Mathematics, 15.01.2021 08:00




World Languages, 15.01.2021 08:10


Mathematics, 15.01.2021 08:10

Mathematics, 15.01.2021 08:10

History, 15.01.2021 08:10






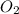
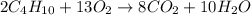
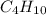 combine with 13 moles of
combine with 13 moles of 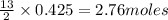 of
of 

