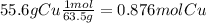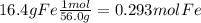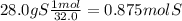Acompound in a copper ore has the following percentage composition by mass:
55.6% copper, 16....

Acompound in a copper ore has the following percentage composition by mass:
55.6% copper, 16.4% iron, 28.0% sulfur.
calculate the empirical formula of the compound.
relative atomic masses (ar): s = 32; fe = 56; cu = 63.5
you must show all of your working.
show all working, x

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 05:00
Agas can holds 2.0 gal of gasoline. what is this quantity in cubic centimeters?
Answers: 2

Chemistry, 22.06.2019 23:30
The density of the solid phase of a substance is 0.90 g/cm3 and the density of the liquid phase is 1.0 g/cm3. a large increase in pressure will a. lower the freezing point b. raise the freezing point c. lower the boiling point d. raise the triple point e. lower the triple point
Answers: 1

Chemistry, 23.06.2019 01:00
Who examines and coordinates the cleanup of polluted sites?
Answers: 2

Chemistry, 23.06.2019 03:00
Is it safe to take 450mg of diphenhydramine hydrochloride?
Answers: 1
You know the right answer?
Questions






Mathematics, 23.11.2020 20:10

English, 23.11.2020 20:10

Health, 23.11.2020 20:10


Mathematics, 23.11.2020 20:10

Mathematics, 23.11.2020 20:10


Mathematics, 23.11.2020 20:10


History, 23.11.2020 20:10

Arts, 23.11.2020 20:10

Mathematics, 23.11.2020 20:10

Mathematics, 23.11.2020 20:10


Advanced Placement (AP), 23.11.2020 20:10