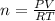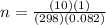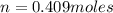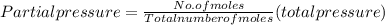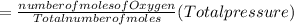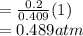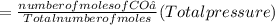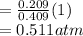Chemistry, 13.10.2019 23:30 jasminelynn135owmyj1
A10 liter flask at 298 k contains a gaseous mixture of o2 and co2 at 1 atmosphere. which statement is true for the partial pressures of o2 and co2 if 0.2 mole of o2 is present in the flask? (given the universal gas constant r = 0.082 l∙atm/k∙mol)

Answers: 3
Another question on Chemistry

Chemistry, 20.06.2019 18:02
How do you find a theoretical mass? is there a difference between theoretical mass and theoretical yield?
Answers: 1

Chemistry, 22.06.2019 04:20
Which formula can be used to calculate the molar mass of ammonia (nh3)? molar mass of n + molar mass of h 3 × molar mass of n + molar mass of h molar mass of n + 3 × molar mass of h 3 × molar mass of n + 3 × molar mass of h
Answers: 1

Chemistry, 22.06.2019 06:30
The following reaction shows sodium carbonate reacting with calcium hydroxide. na2co3 + ca(oh)2 → naoh + caco3 how many grams of naoh are produced from 20.0 grams of na2co3? (molar mass of na = 22.989 g/mol, c = 12.01 g/mol, o = 15.999 g/mol, ca = 40.078 g/mol, h = 1.008 g/mol) 12.2 grams 15.1 grams 24.4 grams 30.2 grams
Answers: 2

You know the right answer?
A10 liter flask at 298 k contains a gaseous mixture of o2 and co2 at 1 atmosphere. which statement i...
Questions

Mathematics, 09.03.2021 01:00



Mathematics, 09.03.2021 01:00

Mathematics, 09.03.2021 01:00







Mathematics, 09.03.2021 01:00



Mathematics, 09.03.2021 01:00

Spanish, 09.03.2021 01:00



World Languages, 09.03.2021 01:00