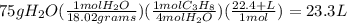Chemistry, 15.11.2019 18:31 yasminnishkan
Given that the molar mass of h2o is 18.02 g/mol, how many liters of propane are required at stp to produce 75 g of h2o from this reaction?

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 13:30
Apush or pull that moves or changes and object when to objects touch
Answers: 2

Chemistry, 22.06.2019 18:30
How many moles of lead are in 1.50 x 10^12 atoms of lead? could you explain the answer as well and not just give it to me i am refreshing for finals and i need to know how to do it
Answers: 3

Chemistry, 22.06.2019 19:30
Estimate the molar mass of the gas that effuses at 1.6 times the effusion rate of carbon dioxide.
Answers: 1

Chemistry, 22.06.2019 20:00
Listenbase your answer to the question on the information below.nuclear radiation is harmful to living cells, particularly to fast-growing cells, such as cancer cells and blood cells. an external beam of the radiation emitted from a radioisotope can be directed on a small area of a person to destroy cancer cells within the body.cobalt-60 is an artificially produced radioisotope that emits gamma rays and beta particles. one hospital keeps a 100.0-gram sample of cobalt-60 in an appropriate, secure storage container for future cancer treatment.which choice represents the correct product for the beta decay of the co-60? fe-60ni-60fe-61ni-61
Answers: 2
You know the right answer?
Given that the molar mass of h2o is 18.02 g/mol, how many liters of propane are required at stp to p...
Questions

Physics, 18.05.2021 17:20


Mathematics, 18.05.2021 17:20


Mathematics, 18.05.2021 17:20


SAT, 18.05.2021 17:20

Chemistry, 18.05.2021 17:20


Mathematics, 18.05.2021 17:20

Mathematics, 18.05.2021 17:20


Mathematics, 18.05.2021 17:20

Mathematics, 18.05.2021 17:20


Mathematics, 18.05.2021 17:20

Mathematics, 18.05.2021 17:20


Mathematics, 18.05.2021 17:20