
Achemist places a 100 g cube of each of the metals listed in the table on a separate hot plate. all of the hot plates are identical. each hot plate is set on medium heat, and all of the cubes have the same initial temperature. based on the specific heat values in the table, rank the metals in terms of how quickly they will reach the target temperature of 80°c. use "1" to indicate the fastest and "5" to indicate the slowest.

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 18:30
In a sample of oxygen gas at room temperature, the average kinetic energy of all the balls stays constant. which postulate of kinetic molecular theory best explains how this is possible?
Answers: 2

Chemistry, 22.06.2019 06:10
How many moles of gas are present if p=11 atm, v=12l, t=185k?
Answers: 1

Chemistry, 22.06.2019 14:50
Consider the following multistep reaction: a b→ab(slow) a ab→a2b(fast)−−−−−−−−−−−−−−−−− 2a b→a2b(overall) based on this mechanism, determine the rate law for the overall reaction. express your answer in standard masteringchemistry format. for example, if the rate law is k[a]3[b]2 type k*[a]^3*[b]^2
Answers: 3

You know the right answer?
Achemist places a 100 g cube of each of the metals listed in the table on a separate hot plate. all...
Questions


Mathematics, 27.04.2021 05:20



English, 27.04.2021 05:20



Mathematics, 27.04.2021 05:20



Mathematics, 27.04.2021 05:20

History, 27.04.2021 05:20


Chemistry, 27.04.2021 05:20

History, 27.04.2021 05:20

Chemistry, 27.04.2021 05:20


Mathematics, 27.04.2021 05:20

History, 27.04.2021 05:20

Physics, 27.04.2021 05:20

 5 is: Pb, Sn, Cu, Fe, Al.
5 is: Pb, Sn, Cu, Fe, Al.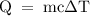
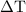 is the change in temperature.
is the change in temperature.

