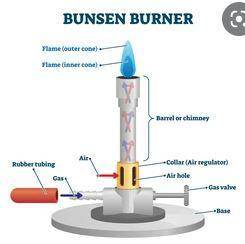
Enter the numbers 1 to 5 to put in order the steps for lighting a bunsen burner.
use a strike...

Chemistry, 17.11.2019 07:31 danielapenaoypgns
Enter the numbers 1 to 5 to put in order the steps for lighting a bunsen burner.
use a strike lighter to light the flame.
turn on the gas supply to the bunsen burner.
clear the area of anything flammable, such as liquids, hair, etc.
adjust the air flow to control the size of the flame.
close the air supply on the bunsen burner.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 12:30
The melting point of sulfur is 115 °c and its boiling point is 445 °c. what state would sulfur be in at 200 °c?
Answers: 1

Chemistry, 23.06.2019 02:10
Detrimental the length of the object shown 1. 97.8mm 2. 97.80 mm 3. 97mm 4. 98mm
Answers: 2

Chemistry, 23.06.2019 03:30
The molar mass of iron(fe) is 55.8 g/mol. what is the mass in grams of 2.25 moles of iron?
Answers: 1

Chemistry, 23.06.2019 06:50
The student repeated the experiment using a higher concentration of acid. the same volume of acid and the same mass of magnesium ribbon were used. what volume of hydrogen gas would have been produced after 60 seconds?
Answers: 1
You know the right answer?
Questions

Mathematics, 30.01.2020 21:45





Mathematics, 30.01.2020 21:45

Mathematics, 30.01.2020 21:45

English, 30.01.2020 21:45








Mathematics, 30.01.2020 21:45


Social Studies, 30.01.2020 21:45

Biology, 30.01.2020 21:45

Biology, 30.01.2020 21:45