
Chemistry, 17.10.2019 03:00 Foxfire5109
How many liters of methane gas (ch4) need to be combusted to produce 8.5 liters of water vapor, if all measurements are taken at the same temperature and pressure? show all of the work used to solve this problem. ch4 (g) + 2o2 (g) yields co2 (g) + 2h2o (g)

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 14:20
Calculate the enthalpy of the following reaction: 4 b (s) + 3 o2 (g) → 2 b2o3 (s) given the following pertinent information: (a) b2o3 (s) + 3 h2o (g) → 3 o2 (g) + b2h6 (g), δhoa = +2035 kj (b) 2 b (s) + 3 h2 (g) → b2h6 (g), δhob = +36 kj (c) h2 (g) + latex: \frac{1}{2} 1 2 o2 (g) → h2o (l), δhoc = −285 kj (d) h2o (l) → h2o (g), δhod = +44 kj
Answers: 3

Chemistry, 22.06.2019 04:00
The rules of engagement (roe) working group is often used to (select all that apply.)
Answers: 2

Chemistry, 22.06.2019 17:30
Observation and experimentation have led many scientists to accept a theory about the origin of the universe. this theory is called the big bang theory. scientific evidence collected and observed by scientists around the world suggests that the universe is ever expanding from a hot and dense initial state. what makes this a scientific theory? (2 points)
Answers: 2

Chemistry, 22.06.2019 21:30
What is the correct name for the compound cocl3? a) cobalt(i) chloride b) cobalt(i) chlorate c) cobalt(ii) chlorate d) cobalt(iii) chloride
Answers: 1
You know the right answer?
How many liters of methane gas (ch4) need to be combusted to produce 8.5 liters of water vapor, if a...
Questions

History, 29.12.2019 15:31


Mathematics, 29.12.2019 15:31

History, 29.12.2019 15:31

Mathematics, 29.12.2019 15:31


Mathematics, 29.12.2019 15:31



Chemistry, 29.12.2019 15:31


Mathematics, 29.12.2019 15:31


Geography, 29.12.2019 15:31

Mathematics, 29.12.2019 15:31

English, 29.12.2019 15:31

English, 29.12.2019 15:31


Geography, 29.12.2019 15:31

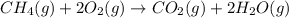
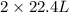 volume of water vapor produced from 22.4 L volume of methane gas
volume of water vapor produced from 22.4 L volume of methane gas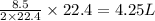 volume of methane gas
volume of methane gas

