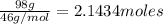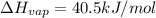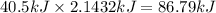Chemistry, 02.10.2019 10:50 moniquejg1800
How much energy is required to vaporize 98.6 g of ethanol (c2h5oh) at its boiling point, if its δhvap is 40.5 kj/mol? how much energy is required to vaporize 98.6 g of ethanol (c2h5oh) at its boiling point, if its δhvap is 40.5 kj/mol? 52.8 kj 11.5 kj 86.7 kj 39.9 kj 18.9 kj

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 19:00
Asmall amount of a solid is added to water. the observation made after fifteen minutes is shown in the figure. which of these solids has been probably added to water? a) oil b) sand c) sugar d) wood chips
Answers: 1


Chemistry, 22.06.2019 13:00
In a copper wire, a temperature increase is the result of which of the following
Answers: 1

Chemistry, 22.06.2019 16:50
Ajet plane is speeding down the runway during takeoff. air resistance is not negligible. identify the forces on the jet.
Answers: 3
You know the right answer?
How much energy is required to vaporize 98.6 g of ethanol (c2h5oh) at its boiling point, if its δhva...
Questions

History, 26.06.2019 13:00







Mathematics, 26.06.2019 13:00



Chemistry, 26.06.2019 13:00



Mathematics, 26.06.2019 13:00



Mathematics, 26.06.2019 13:00


Mathematics, 26.06.2019 13:00

Mathematics, 26.06.2019 13:00