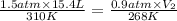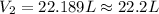Chemistry, 10.12.2019 04:31 Smartpotato9555
Agas has an initial volume of 15.4 l at a pressure of 1.5 atm and a temperature of 310 k. the pressure of the gas decreases to 0.9 atm as the temperature decreases to 268 k. what is the final volume of the gas? round your answer to the nearest tenth.
22.2 l

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 02:30
Which compound contains both ionic and covalent bonds? a) hbr b)cbr4 c)nabr d) naoh
Answers: 2

Chemistry, 22.06.2019 05:30
What is the mass defect of a mole of nuclei with 1.8 x 10^15 j/mol binding energy?
Answers: 1

Chemistry, 22.06.2019 06:10
Explain the relationship between forward and backward reactions in equilibrium, and predict how changing the amount of a reactant (creating a tension) will affect that relationship.
Answers: 1

Chemistry, 22.06.2019 11:00
Freezing and boiling are endothermic processes. this means that these processes absorb energy from their surroundings in order to occur. use this information and the data you collected in the phase change gizmo to describe what happens to the temperature of water when you boil it, then explain why this result occurs.
Answers: 1
You know the right answer?
Agas has an initial volume of 15.4 l at a pressure of 1.5 atm and a temperature of 310 k. the pressu...
Questions


Medicine, 03.05.2022 01:00




Business, 03.05.2022 01:10

Spanish, 03.05.2022 01:20



Arts, 03.05.2022 01:30






Biology, 03.05.2022 02:00


Mathematics, 03.05.2022 02:20


Mathematics, 03.05.2022 02:30

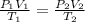
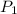 = initial pressure of gas = 1.5 atm
= initial pressure of gas = 1.5 atm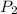 = final pressure of gas = 0.9 atm
= final pressure of gas = 0.9 atm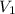 = initial volume of gas = 15.4 L
= initial volume of gas = 15.4 L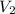 = final volume of gas = ?
= final volume of gas = ?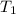 = initial temperature of gas = 310 K
= initial temperature of gas = 310 K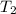 = final temperature of gas = 268 K
= final temperature of gas = 268 K