
Chemistry, 18.11.2019 07:31 esdoles3865
If 5.65 grams of zinc metal react with 21.6 grams of silver nitrate, how many grams of silver metal can be formed and how many grams of the excess reactant will be left over when the reaction is complete? show all of your work.
unbalanced equation: zn + agno3 zn(no3)2 + ag

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 04:20
Which formula can be used to calculate the molar mass of ammonia (nh3)? molar mass of n + molar mass of h 3 × molar mass of n + molar mass of h molar mass of n + 3 × molar mass of h 3 × molar mass of n + 3 × molar mass of h
Answers: 1

Chemistry, 22.06.2019 11:50
Which of the following statements about hybrid orbitals is or are true? choose all that apply. choose all that apply. under sp2 hybridization, the large lobes point to the vertices of an equilateral triangle. after an atom undergoes sp hybridization there is one unhybridized p orbital on the atom. the angle between the large lobes of sp3 hybrids is 109.5∘
Answers: 2

Chemistry, 22.06.2019 14:00
In the space, show a correct numerical setup for calculating the number of moles of co2 present in 11 grams of co2
Answers: 1

Chemistry, 22.06.2019 19:10
How does the atmosphere to make earth livable? check all that apply. causes the seasons contains oxygen provides warmth creates important nutrients blocks harmful energy from the sun plz like !
Answers: 2
You know the right answer?
If 5.65 grams of zinc metal react with 21.6 grams of silver nitrate, how many grams of silver metal...
Questions

History, 22.09.2019 19:30



Chemistry, 22.09.2019 19:30



History, 22.09.2019 19:30

History, 22.09.2019 19:30

Chemistry, 22.09.2019 19:30


Mathematics, 22.09.2019 19:30




Mathematics, 22.09.2019 19:30





Mathematics, 22.09.2019 19:30

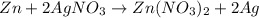
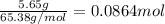
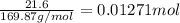
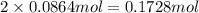 silver nitrate
silver nitrate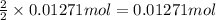 of silver metal
of silver metal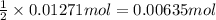 of zinc
of zinc

