
Chemistry, 24.10.2019 18:43 kenyakids405
In the following combustion reaction of acetylene (c2h2), how many liters of co2 will be produced if 60 liters of o2 is used, given that both gases are at stp? 2c2h2+5o2 2h2o+4co2 the volume of one mole of gas at stp is 22.4 liters. 48 liters 0.02 liters 240 liters 300 liters nextreset

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 14:50
What type of reaction is illustrated? 2c12o5 = 2cl2 + 502
Answers: 2

Chemistry, 22.06.2019 00:00
Several kinds of bears are found on earth. most bears are brown or black, but one type of bear, the polar bear, is white. what process led to this difference in fur color? explain your answer.
Answers: 1


Chemistry, 22.06.2019 11:30
Which statement best describes the flow of energy in this scenario
Answers: 1
You know the right answer?
In the following combustion reaction of acetylene (c2h2), how many liters of co2 will be produced if...
Questions


History, 12.02.2020 02:14

Mathematics, 12.02.2020 02:14


Mathematics, 12.02.2020 02:14



Computers and Technology, 12.02.2020 02:15



Computers and Technology, 12.02.2020 02:15









Mathematics, 12.02.2020 02:15

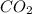 produced will be, 48 liters.
produced will be, 48 liters.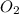 = 60 L
= 60 L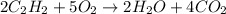
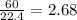 mole of
mole of 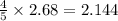 moles of
moles of 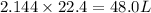 volume of
volume of 

