Chemistry, 29.08.2019 14:30 avahrider1
The pressure of 5.00 l of gas increases from 1.50 atm to 1240 mmhg. what is the final volume of the gas, assuming constant temperature?

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 12:10
|using the periodic tablewarm-upuse the periodic table in the tools bar to answer the following questions.what elemental classification does oxygen belongto? done
Answers: 3


Chemistry, 22.06.2019 19:50
When the mercury level in a barometer decreases that atmospheric pressure has
Answers: 3

Chemistry, 22.06.2019 20:10
What would happen to a volleyball left outside in the winter? o o o o a. it would expand. b. it would lose air. c. it would shrink. d. it would explode.
Answers: 2
You know the right answer?
The pressure of 5.00 l of gas increases from 1.50 atm to 1240 mmhg. what is the final volume of the...
Questions


Physics, 13.12.2020 20:20


Arts, 13.12.2020 20:30

English, 13.12.2020 20:30

Mathematics, 13.12.2020 20:30


Advanced Placement (AP), 13.12.2020 20:30





Chemistry, 13.12.2020 20:30

Computers and Technology, 13.12.2020 20:30

Mathematics, 13.12.2020 20:30


Chemistry, 13.12.2020 20:30

Computers and Technology, 13.12.2020 20:30

Mathematics, 13.12.2020 20:30

Chemistry, 13.12.2020 20:30

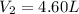
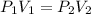
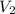 as required, including the conversion from mmHg to atm for the final pressure:
as required, including the conversion from mmHg to atm for the final pressure: