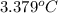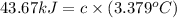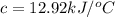Chemistry, 26.09.2019 22:40 kraigstlistt
At constant volume, the heat of combustion of a particular compound, compound a, is –3568.0 kj/mol. when 1.411 g of compound a (molar mass = 115.27 g/mol) was burned in a bomb calorimeter, the temperature of the calorimeter (including its contents) rose by 3.379 °
c. using this data, what is the heat capacity (calorimeter constant) of the calorimeter?

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 01:30
If 34.2 grams of lithium react with excess water, how many liters of hydrogen gas can be produced at 299 kelvin and 1.21 atmospheres? 2 li (s) + 2 h2o (l) yields 2 lioh (aq) + h2 (g)
Answers: 3

Chemistry, 22.06.2019 09:20
How have the greenhouse gasses increased from the year 2000 to 2018
Answers: 2

Chemistry, 22.06.2019 12:00
What is the percentage of hydrogen in nitrogen trihydride
Answers: 1

Chemistry, 22.06.2019 12:30
Sodium sulfate dissolves as follows: na2so4(s) → 2na+(aq) + so42- (aq). how many moles of na2so4 are required to make 1.0 l of solution in which the na concentration is 0.10 m?
Answers: 2
You know the right answer?
At constant volume, the heat of combustion of a particular compound, compound a, is –3568.0 kj/mol....
Questions

Biology, 20.10.2021 22:10

Arts, 20.10.2021 22:10

Chemistry, 20.10.2021 22:10





Social Studies, 20.10.2021 22:20



Mathematics, 20.10.2021 22:20


Mathematics, 20.10.2021 22:20



Chemistry, 20.10.2021 22:20



Computers and Technology, 20.10.2021 22:20

Computers and Technology, 20.10.2021 22:20

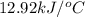
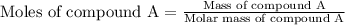
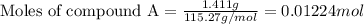
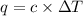
 = change in temperature =
= change in temperature =