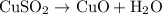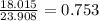Chemistry, 16.09.2019 18:40 juicemankinnie95
In the decomposition reaction, 1 mole of water (mw = 18.015 g/mol) was produced for every mole of cuo (mw = 79.545 g/mol) produced. given that 3.327 g of cuo was produced during the reaction, how many grams of water were released as water vapor? (number of moles = mass (g) / molar mass (g/ choose the closest answer.

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 19:00
Iknow the answer to 13 is b and 14 is d. i just need to know why the correct answers are correct
Answers: 1

Chemistry, 22.06.2019 22:50
At the current rate, a graph of carbon dioxide produced by fossil fuels over time would slope upward slope downward be horizontal be vertical
Answers: 3

Chemistry, 23.06.2019 07:40
What is the reduction potential of a hydrogen electrode that is still at standard pressure, but has ph = 5.65 , relative to the she?
Answers: 1

Chemistry, 23.06.2019 08:10
An experiment is conducted to see if cats preferred skim milk or 2% milk. a cup of skim milkwas put out for 5 kittens and then measured how much the kittens drank over the course of aday. following a cup of 2% milk was purout for the skittens and then masured how much thekittens drank over the course of a day. the same kittens were used and the milk was served atthe same temperature. it was discovered that the cats liked the 2% milk more than the skimmilk. what is the dependent variable in this experiment?
Answers: 1
You know the right answer?
In the decomposition reaction, 1 mole of water (mw = 18.015 g/mol) was produced for every mole of cu...
Questions



Mathematics, 25.10.2019 00:43

History, 25.10.2019 00:43


History, 25.10.2019 00:43

History, 25.10.2019 00:43

Business, 25.10.2019 00:43

Mathematics, 25.10.2019 00:43



Mathematics, 25.10.2019 00:43


Mathematics, 25.10.2019 00:43

Mathematics, 25.10.2019 00:43

Mathematics, 25.10.2019 00:43

Mathematics, 25.10.2019 00:43


Mathematics, 25.10.2019 00:43

English, 25.10.2019 00:43