
Chemistry, 02.10.2019 10:10 terrasami2330
In the reaction mg(s) + 2hcl(aq) → h2(g) + mgcl2(aq), how many moles of hydrogen gas will be produced from 250.0 milliliters of a 3.0 m hcl in an excess of mg? 0.75 moles 0.38 moles 3.0 moles 1.5 moles

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 18:30
For each of the following mixtures decide if filtering would be suitable to separate the substances. explain your answers. oil in water sugar in water sand in water chalk in water tea leaves in a cup of tea
Answers: 2

Chemistry, 22.06.2019 01:30
Aroller coaster car is traveling down a track at 22 m/s. the car has a mass of 2000 kg. what is the kinetic energy of the car? a) 22,000 j b) 968,000 j c) 484,000 j d) 44,000 j
Answers: 2

Chemistry, 22.06.2019 08:30
If i initially have a gas at a pressure of 12 atm, a volume of 23 liters, and a temperature of 200 k, and then i raise the pressure to 14 atm and increase the temperature to 300 k, what is the new volume of the gas?
Answers: 1

Chemistry, 22.06.2019 10:00
The tendency of water molecules to stick together is referred to as a) adhesion b) polarity c) cohesion d) transpiration e) evaporation
Answers: 1
You know the right answer?
In the reaction mg(s) + 2hcl(aq) → h2(g) + mgcl2(aq), how many moles of hydrogen gas will be produce...
Questions


Mathematics, 11.11.2020 01:00

English, 11.11.2020 01:00

History, 11.11.2020 01:00




Mathematics, 11.11.2020 01:10


Mathematics, 11.11.2020 01:10

Geography, 11.11.2020 01:10




Physics, 11.11.2020 01:10

Mathematics, 11.11.2020 01:10


Mathematics, 11.11.2020 01:10


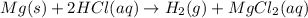
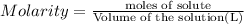
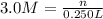
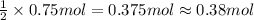 of hydrogen gas.
of hydrogen gas.

