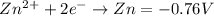Chemistry, 16.10.2019 12:30 alisaharnauth
Which half reaction is most easily oxidized?
ag+ + e− → ag = 0.80 v
br2 + 2e− → 2br− = 1.07 v
fe3+ + e− → fe2+ = 0.77 v
zn2+ + 2e− → zn = –0.76 v

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 02:30
Asa choose the correct set of reaction coefficients to properly balance the following chemical equation according to the law of conservation of mass: __s8 + __o2 ==> __so2 1, 1, 8 1, 8, 1 1, 8, 8 8, 1, 1
Answers: 1

Chemistry, 22.06.2019 12:30
According to the valence shell electron pair repulsion (vsepr) theory, a molecule that has four electron groups around the central atom will exhibit what electron geometry? view available hint(s) according to the valence shell electron pair repulsion (vsepr) theory, a molecule that has four electron groups around the central atom will exhibit what electron geometry? trigonal bipyramidal tetrahedral square planar determination of electron geometry requires information on whether the electron groups are lone pairs or bonding groups.
Answers: 2


You know the right answer?
Which half reaction is most easily oxidized?
ag+ + e− → ag = 0.80 v
br2 + 2...
ag+ + e− → ag = 0.80 v
br2 + 2...
Questions

English, 22.10.2021 14:00



Chemistry, 22.10.2021 14:00

Mathematics, 22.10.2021 14:00



Mathematics, 22.10.2021 14:00

English, 22.10.2021 14:00

History, 22.10.2021 14:00


Mathematics, 22.10.2021 14:00

Biology, 22.10.2021 14:00

History, 22.10.2021 14:00




History, 22.10.2021 14:00

Mathematics, 22.10.2021 14:00