
Chemistry, 20.09.2019 14:00 masonprice
A0.00100 mol sample of ca(oh)2 requires 25.00 ml of aqueous hcl for neutralization according to the reaction below. what is the concentration of the hcl? ca(oh)2(s) + 2hcl(aq) --> cacl2(aq) + h2o(l)

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 14:00
Which of the following statements is true? question 4 options: nuclear decay rates vary with the conditions of the reaction, but chemical reaction rates do not. chemical reaction rates vary with the conditions of the reaction, but nuclear decay rates do not. neither chemical reaction rates nor nuclear decay rates vary with the conditions of the reaction. both chemical reaction rates and nuclear decay rates vary with the conditions of the reaction.
Answers: 1


Chemistry, 22.06.2019 05:00
In 1901, thomas edison invented the nickel-iron battery. the following reaction takes place in the battery. fe(s) + 2 nio(oh)(s) + 2 h2o(l) fe(oh)2(s) + 2 ni(oh)2(aq) how many mole of fe(oh)2, is produced when 5.35 mol fe and 7.65 mol nio(oh) react?
Answers: 1

You know the right answer?
A0.00100 mol sample of ca(oh)2 requires 25.00 ml of aqueous hcl for neutralization according to the...
Questions



Mathematics, 15.04.2021 18:00

English, 15.04.2021 18:00

Spanish, 15.04.2021 18:00



Computers and Technology, 15.04.2021 18:00


Geography, 15.04.2021 18:00




Business, 15.04.2021 18:00



Mathematics, 15.04.2021 18:00

History, 15.04.2021 18:00

Mathematics, 15.04.2021 18:00

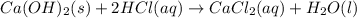
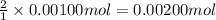
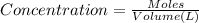
![[HCl]=\frac{0.00200 mol}{0.025 L}=0.08 mol/L](/tpl/images/0246/4244/941e7.png)


