
Consider the following element combinations. classify the bonds formed between each pair as ionic, polar covalent, or nonpolar covalent qualitatively based solely on each element's position on the periodic table? do not conduct calculations. 1. rb and i 2. br and br 3. s and i 4. n and n 5. o and f 6. cs and cl 7. ca and o 8. p and i

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 01:40
C3h8o3 - glycerol major species present when dissolved in water
Answers: 2

Chemistry, 22.06.2019 06:30
This drawing shows a human body system. what is the primary function of this body system?
Answers: 3

Chemistry, 23.06.2019 01:00
If a straight-chain hydrocarbon is a gas at room temperature, how many carbon atoms will it have? a. 6 carbon atoms b. 12 carbon atoms c. 24 carbon atoms d. 3 carbon atoms
Answers: 1

Chemistry, 23.06.2019 01:30
Which conclusion fits the data in the table? a. heat chemically changes chocolate and margarine. b. all solids become liquid at 100°f. c. removing heat from a substance it to melt. d. matter may change shape when it is heated.
Answers: 1
You know the right answer?
Consider the following element combinations. classify the bonds formed between each pair as ionic, p...
Questions

Mathematics, 22.05.2020 18:01


Mathematics, 22.05.2020 18:01

Mathematics, 22.05.2020 18:01




Mathematics, 22.05.2020 18:01

English, 22.05.2020 18:01

History, 22.05.2020 18:01

Mathematics, 22.05.2020 18:01


Biology, 22.05.2020 18:01

Engineering, 22.05.2020 18:01

English, 22.05.2020 18:01



Mathematics, 22.05.2020 18:01


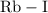 is an
is an 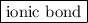 .
.
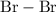 is a
is a 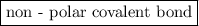 .
.
 is a
is a 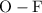 is a
is a 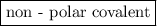 .
.
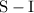 is a
is a  is an
is an  is a
is a 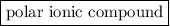 .
.
 is a
is a 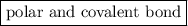 .
.
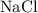 is an ionic compound.
is an ionic compound.
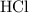 . Here the bond is polar and covalent. Another example of covalent molecule is methane where the difference in the C-H electronegativities is very small so it regarded as a pure covalent molecule.
. Here the bond is polar and covalent. Another example of covalent molecule is methane where the difference in the C-H electronegativities is very small so it regarded as a pure covalent molecule.
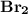 is a non-polar covalent bond. Similarly
is a non-polar covalent bond. Similarly 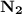 is a non-polar covalent molecule.
is a non-polar covalent molecule.
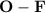 bond are non-polar covalent bonds.
bond are non-polar covalent bonds.
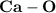 is a polar covalent bond.
is a polar covalent bond.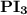 is regarded as polar and covalent bonds.
is regarded as polar and covalent bonds.



