
Chemistry, 27.10.2019 14:43 luckilyalexa
Phosphorus trichloride, pcl3, decomposes to form elemental phosphorus and chlorine. the equation is: 4pcl3 → p4 + 6cl2. balance the equation and determine the mass in grams of chlorine that would be formed if 25 grams of pcl3 (molecular mass = 137.32 g/mol) decompose. grams of cl2

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 03:30
At a temperature of 393 k, the temperature of a sample of nitrogen is 1.07 atm what will the pressure be at a temperature of 478 k
Answers: 1

Chemistry, 22.06.2019 13:00
Asubstance is a good conductor of electricity which of the following best explains a probable position of the substance in a periodic table
Answers: 3

Chemistry, 22.06.2019 18:00
Alidded glass container is filled with a colored gas. after a period of time, it is observed that the gas is uniformly spread throughout the box and that the movement has slowed considerably. next, a warm iron plate is carefully placed under the box. why is there resumed movement of the gas in the container?
Answers: 2

Chemistry, 22.06.2019 18:30
How many moles of lead are in 1.50 x 10^12 atoms of lead? could you explain the answer as well and not just give it to me i am refreshing for finals and i need to know how to do it
Answers: 3
You know the right answer?
Phosphorus trichloride, pcl3, decomposes to form elemental phosphorus and chlorine. the equation is:...
Questions














Health, 16.01.2021 16:50

Mathematics, 16.01.2021 16:50



Mathematics, 16.01.2021 16:50


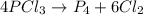
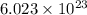 of particles.
of particles.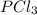 decompose to give 6 moles of
decompose to give 6 moles of 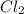
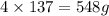 of
of 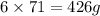 of
of 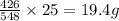 of
of 

