
Chemistry, 02.10.2019 00:30 myasiaspencer
Did the rolaids solution function as a good buffer? justify your results.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 12:00
Consider the following reaction at equilibrium. 2co2 (g) 2co (g) + o2 (g) h° = -514 kj le châtelier's principle predicts that the equilibrium partial pressure of co (g) can be maximized by carrying out the reaction a. at high temperature and high pressure b. at high temperature and low pressure c. at low temperature and low pressure d. at low temperature and high pressure e. in the presence of solid carbon
Answers: 2

Chemistry, 22.06.2019 23:30
If maltose undergoes hydrolysis what subunits does it results to?
Answers: 2


Chemistry, 23.06.2019 00:30
What is the percent by mass of magnesium sulfate in mgso4.7h2o
Answers: 3
You know the right answer?
Did the rolaids solution function as a good buffer? justify your results....
Questions

Mathematics, 30.03.2020 20:45







Mathematics, 30.03.2020 20:45

Social Studies, 30.03.2020 20:45

English, 30.03.2020 20:45





Biology, 30.03.2020 20:45

Mathematics, 30.03.2020 20:45

English, 30.03.2020 20:45


History, 30.03.2020 20:45

English, 30.03.2020 20:45

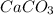 ) and magnesium hydroxide(
) and magnesium hydroxide(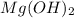 . So, this rolaid solution being alkaline (basic) in nature can just neutralize the excess acid present. It can act as a buffer by absorbing excess
. So, this rolaid solution being alkaline (basic) in nature can just neutralize the excess acid present. It can act as a buffer by absorbing excess 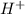 ions and maintaining the pH upon the addition of acid. But in a broader sense, Rolaids solution cannot act as a buffer in all conditions as it can only absorb the excess acid but has no effect when excess base is added to a solution.
ions and maintaining the pH upon the addition of acid. But in a broader sense, Rolaids solution cannot act as a buffer in all conditions as it can only absorb the excess acid but has no effect when excess base is added to a solution.

