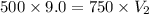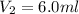Chemistry, 20.09.2019 01:00 daemonacoster
Asample of gas occupies 9.0 ml at a pressure of 500.0 mm hg. a new volume of the same sample is at a pressure of 750.0 mm hg. is the new pressure smaller or larger than the original? therefore, is the new volume larger or smaller than 9.0 ml? what is this new volume?

Answers: 1
Another question on Chemistry


Chemistry, 22.06.2019 19:30
Which one of the following substances would be the most soluble in ccl4? na2so4 h2o ch3ch2ch2ch2oh c4h10 hi
Answers: 1

Chemistry, 22.06.2019 20:10
The lattice enthalpy (formation of ionic solid from ions in the gas phase) for agcl(s) is -916 kj/mol and the hydration enthalpy (dissolution of gaseous ions into water) is -850 kj/mol. how much heat (in joules) is involved in forming 1l of saturated agcl solution (1.8 × 10-4 g / 100 ml water) by dissolving agcl(s)? assume solution volume does not change much upon dissolution. the equations are given below. ag+(g) + cl−(g) æ agcl(s)
Answers: 3

Chemistry, 22.06.2019 22:40
Percent ionization for a weak acid (ha) is determined by the following formula: percent ionization=[ha] ionized[ha] initial×100%for strong acids, ionization is nearly complete (100%) at most concentrations. however, for weak acids, the percent ionization changes significantly with concentration. the more diluted the acid is, the greater percent ionization.a certain weak acid, ha, has a ka value of 9.4×10? 7.part acalculate the percent ionization of ha in a 0.10 m solution.part bcalculate the percent ionization of ha in a 0.010 m solution
Answers: 1
You know the right answer?
Asample of gas occupies 9.0 ml at a pressure of 500.0 mm hg. a new volume of the same sample is at a...
Questions

Mathematics, 19.10.2019 00:30


Mathematics, 19.10.2019 00:30


History, 19.10.2019 00:30



Mathematics, 19.10.2019 00:30

Mathematics, 19.10.2019 00:30



History, 19.10.2019 00:30



Mathematics, 19.10.2019 00:30


Mathematics, 19.10.2019 00:30

History, 19.10.2019 00:30

World Languages, 19.10.2019 00:30

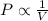 (At constant temperature and number of moles)
(At constant temperature and number of moles)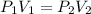
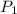 = initial pressure of gas = 500.0 mmHg
= initial pressure of gas = 500.0 mmHg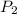 = final pressure of gas= 750.0 mmHg
= final pressure of gas= 750.0 mmHg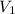 = initial volume of gas = 9.0 ml
= initial volume of gas = 9.0 ml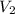 = final volume of gas = ?
= final volume of gas = ?