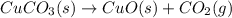Chemistry, 31.08.2019 12:20 donmak3833
When heated, solid copper(ii) carbonate decomposes to solid copper(ii) oxide and carbon dioxide gas. give the balanced chemical equation (including phases) that describes this reaction?

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 16:00
What is lincoln's purpose in writing this speech? question 1 options: to stress the difficulties of war to honor those who died in the war to call for an end to the war to call the country to join a new war
Answers: 1

Chemistry, 21.06.2019 23:00
When determining the shape of a molecule, it is important to draw a lewis dot structure first in order to see the total number a. electrons within the moleculeb. bonding and unshared pairs around central atomc. unshared pair within the molecule( i really need it )
Answers: 1

Chemistry, 22.06.2019 08:30
Analyze how limestone is weathered and identify the features that are formed as a result of this dissolution
Answers: 1

Chemistry, 22.06.2019 12:30
Sodium sulfate dissolves as follows: na2so4(s) → 2na+(aq) + so42- (aq). how many moles of na2so4 are required to make 1.0 l of solution in which the na concentration is 0.10 m?
Answers: 2
You know the right answer?
When heated, solid copper(ii) carbonate decomposes to solid copper(ii) oxide and carbon dioxide gas....
Questions


Business, 11.03.2022 01:00


Mathematics, 11.03.2022 01:00


Chemistry, 11.03.2022 01:00

Biology, 11.03.2022 01:00

Mathematics, 11.03.2022 01:00

Computers and Technology, 11.03.2022 01:00

Biology, 11.03.2022 01:00




Social Studies, 11.03.2022 01:00



Mathematics, 11.03.2022 01:00


English, 11.03.2022 01:00