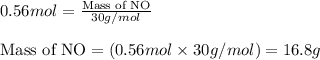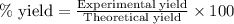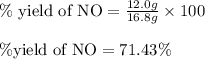Chemistry, 31.08.2019 22:30 johndiaz26
Nitric oxide, no, is made from the oxidation of nh3, and the reaction is represented by the equation 4nh3 + 5o2 ? 4no + 6h2o an 9.5-g sample of nh3 gives 12.0 g of no. the percent yield of no is :

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 04:30
Suppose that during that icy hot lab 65,000 j of energy were transferred to 450 g of water at 20°c what would have have been the final temperature of the water
Answers: 2

Chemistry, 22.06.2019 06:00
What type of electromagnetic radiation has a shorter wavelength than blue light
Answers: 2

Chemistry, 22.06.2019 12:00
Give the set of reactants (including an alkyl halide and a nucleophile) that could be used to synthesize the following ether: draw the molecules on the canvas by choosing buttons from the tools (for bonds and charges), atoms, and templates toolbars, including charges where needed. ch3ch2och2ch2chch3 | ch3
Answers: 1

Chemistry, 22.06.2019 18:00
Chlorophyll a had the molecular formula c55h72mgn4o5 how many atoms are in this molecule
Answers: 2
You know the right answer?
Nitric oxide, no, is made from the oxidation of nh3, and the reaction is represented by the equation...
Questions


Mathematics, 23.08.2021 22:40


Computers and Technology, 23.08.2021 22:40















Computers and Technology, 23.08.2021 22:40

World Languages, 23.08.2021 22:40

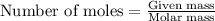 .....(1)
.....(1)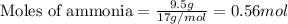
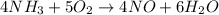
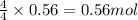 of NO
of NO