
If 2.0 moles of a and 3.0 moles of b react according to the hypothetical reaction below, how many moles of the excess reactant will be left over at the end of the reaction? a + 2b → ab2 (3 points) 1.0 mol b left over 0.50 mol a left over 1.5 mol b left over 1.5 mol a left over

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 18:30
Strong conductivity of plasma allows it to act and react as and
Answers: 2

Chemistry, 22.06.2019 09:10
How have the greenhouse gasses increased from the year 2000 to 2018
Answers: 2

Chemistry, 22.06.2019 13:30
Which of the following natural processes is most likely to support the formation of an underwater sinkhole? a pollution buildup from deposited minerals b limestone cave collapsing due to changes in sea level c erosion of large amounts of sand moved by ocean waves d oxidation of rock formed by chemical weathering
Answers: 1

Chemistry, 22.06.2019 16:00
What rule is used to determine how many covalent bonds an element can form? a. the number of covalent bonds is equal to six c the number of covalent bonds is equal to five minus the group number plus the group number b. the number of covalent bonds is equal to eight d. none of the above minus the group number select the best answer from the choices provided
Answers: 2
You know the right answer?
If 2.0 moles of a and 3.0 moles of b react according to the hypothetical reaction below, how many mo...
Questions



Mathematics, 31.01.2021 22:30

English, 31.01.2021 22:30



Mathematics, 31.01.2021 22:30

Mathematics, 31.01.2021 22:30

English, 31.01.2021 22:30

Mathematics, 31.01.2021 22:30

English, 31.01.2021 22:30



Mathematics, 31.01.2021 22:30

Mathematics, 31.01.2021 22:30

Mathematics, 31.01.2021 22:30


Mathematics, 31.01.2021 22:30

Health, 31.01.2021 22:30

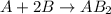
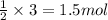 of A.
of A.


