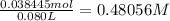Answers: 1
Another question on Chemistry


Chemistry, 22.06.2019 09:00
Suppose you have designed a new thermometer called the x thermometer. on the x scale the boiling point of water is 129 ? x and the freezing point of water is 13 ? x. part a at what temperature are the readings on the fahrenheit and x thermometers the same?
Answers: 1

Chemistry, 22.06.2019 13:50
Read the chemical equation. 2c2h2 + 5o2 → 4co2 + 2h2o which of the following statements would be correct if one mole of c2h2 was used in this reaction? one mole of oxygen was used in this reaction. five moles of oxygen were used in this reaction. four moles of carbon dioxide were produced from this reaction. two moles of carbon dioxide were produced from this reaction.
Answers: 3

Chemistry, 23.06.2019 00:00
How many atoms or molecules are there in a mole of a substance?
Answers: 1
You know the right answer?
Nitric acid + mg(no3)35.0 ml of 0.255 m nitric acid is added to 45.0 ml of 0.328 m mg(no3)2. what is...
Questions

Mathematics, 10.04.2021 05:40

Mathematics, 10.04.2021 05:40


Mathematics, 10.04.2021 05:40



Arts, 10.04.2021 05:40


Computers and Technology, 10.04.2021 05:40


Mathematics, 10.04.2021 05:40

Chemistry, 10.04.2021 05:40

Social Studies, 10.04.2021 05:40

Mathematics, 10.04.2021 05:40

Physics, 10.04.2021 05:40


Mathematics, 10.04.2021 05:40

Chemistry, 10.04.2021 05:40

Mathematics, 10.04.2021 05:40

History, 10.04.2021 05:40

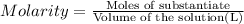
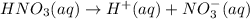
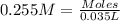
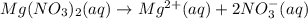
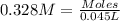
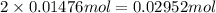 of nitrate ions
of nitrate ions