Why is water considered a polar molecule?
the molecule has no overall charge.
the...


Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 00:00
Which actions would increase the rate at salt dissolves in water? stir the water? crush the salt? use less water? heat the water? cool the salt
Answers: 3


Chemistry, 22.06.2019 06:00
When a spring is compressed, the energy changes from kinetic to potential. which best describes what is causing this change?
Answers: 3

Chemistry, 22.06.2019 15:30
How does a large body of water, such as the ocean, influence climate?
Answers: 1
You know the right answer?
Questions





Mathematics, 17.11.2020 01:00


Mathematics, 17.11.2020 01:00

Mathematics, 17.11.2020 01:00


Mathematics, 17.11.2020 01:00


English, 17.11.2020 01:00

Mathematics, 17.11.2020 01:00



History, 17.11.2020 01:00



Mathematics, 17.11.2020 01:00

English, 17.11.2020 01:00

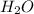 . As oxygen is more electronegative than hydrogen atom hence, it will attract the electrons more towards itself.
. As oxygen is more electronegative than hydrogen atom hence, it will attract the electrons more towards itself. 

