
Chemistry, 01.10.2019 17:00 jet0120996
Magnesium burns in air with a dazzling brilliance to produce magnesium oxide: 2mg(s) + o2(g) ? 2mgo(s) when 4.00 g of magnesium burns, the theoretical yield of magnesium oxide is

Answers: 1
Another question on Chemistry


Chemistry, 22.06.2019 00:00
The p sub shell can hold up to 8 electrons in an atom. true or false?
Answers: 1

Chemistry, 22.06.2019 04:20
Which formula can be used to calculate the molar mass of ammonia (nh3)? molar mass of n + molar mass of h 3 × molar mass of n + molar mass of h molar mass of n + 3 × molar mass of h 3 × molar mass of n + 3 × molar mass of h
Answers: 1

Chemistry, 22.06.2019 12:30
What metric units would you use to measure the thickness of a key
Answers: 3
You know the right answer?
Magnesium burns in air with a dazzling brilliance to produce magnesium oxide: 2mg(s) + o2(g) ? 2mg...
Questions


Mathematics, 04.11.2020 19:50



History, 04.11.2020 19:50

Mathematics, 04.11.2020 19:50



Biology, 04.11.2020 19:50

Mathematics, 04.11.2020 19:50


Chemistry, 04.11.2020 19:50


Mathematics, 04.11.2020 19:50

Spanish, 04.11.2020 19:50



History, 04.11.2020 19:50


 .....(1)
.....(1)
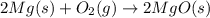
 of magnesium oxide
of magnesium oxide


