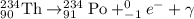Chemistry, 17.09.2019 17:50 ayoismeisalex
Fiestaware is radioactive because the glaze used contains uranium oxide. the half-life of the uranium-238 isotope present in these dishes is about 4.5 billion years. this implies that most of the uranium-238 is still in the dishes and very little has decayed to emit ionizing radiation. uranium-238 initially decays into thorium-234 and an alpha particle as shown in the background, but thorium-234 can further decay to emit beta particles and gamma radiation. what is the decay reaction of thorium-234?

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 09:00
This chart lists four kinds of polymers and their sources. what can be known about all four polymers, despite their differences? they come from living things. they share ionic carbon bonds. they are at least 100 monomers long. they are made of repeating subunits.
Answers: 2

Chemistry, 23.06.2019 01:00
If a sample of radioactive isotopes takes 600 minutes to decay from 400 grams to 50 grams, what is the half-life of the isotope?
Answers: 1

Chemistry, 23.06.2019 03:30
If you need to add 27.50ml of a solution, which piece of glassware would you use to deliver this volume and explain how you would determine if the 27.50 ml was measured?
Answers: 1

Chemistry, 23.06.2019 04:00
If you are told to get 100 ml of stock solution to use to prepare smaller size sample for an experiment, which piece of glassware would you use?
Answers: 3
You know the right answer?
Fiestaware is radioactive because the glaze used contains uranium oxide. the half-life of the uraniu...
Questions




Mathematics, 19.11.2020 20:30






Mathematics, 19.11.2020 20:30



History, 19.11.2020 20:30

History, 19.11.2020 20:30

Social Studies, 19.11.2020 20:30


History, 19.11.2020 20:30

Computers and Technology, 19.11.2020 20:30

Biology, 19.11.2020 20:30